Mind Medicine (MindMed) Inc. (NEO:MMED) (OTCQB:MMEDF) (FRA:BGHM) is Already Preparing TWO Potential Breakthrough Products for Phase II Clinical Trials in 2020
In the 1970s, a relatively small pharmaceutical company called Eli Lilly developed an antidepressant drug. It was the first major treatment for depression … and ushered in a new era of mental health and created a new asset class of medicines.
The drug went on to become the company’s first multibillion-dollar drug and its best-seller from 1992-2001.
Today, it is one of only three drugs deemed an “essential medicine” by the World Health Organization[1], and is still a leading antidepressant—a true household name.
Clearly, Eli Lilly capitalized on being the first to market for a major mental health need.
In 2019, just in the United States, a shockingly large amount of $225 Billion was spent on mental health services. This is a really big market ripe for disruptive medicines and therapies.
And with current lockdowns and stay-at-home orders still in place in markets all around the world, all types of mental health and addiction are going to skyrocket. In fact, according to one scientific study, the consumption of addictive substances can increase 19 times in isolation. [2]
Due to increased anxiety during the Great Lockdown, Xanax prescriptions increased 14.5% during the month of March alone. [3]
We are on the verge of the Great Depression of Mental Health and Addiction.
40 million Americans have lost their jobs since COVID-19 started. When someone loses a job they are more likely to develop addiction and mental health issues..[4]
Answering this are innovative pharmaceutical companies seeking an edge by clearly thinking outside the box, and coming from sources previously seen as taboo and unthinkable… PSYCHEDELICS.
Since 2018, the US Food and Drug Administration (FDA) has granted “breakthrough therapy” status three times to psychedelics-based treatments for major mental health illnesses.[5]
Pharma giant Johnson & Johnson was the first pharmaceutical developer to receive the FDA’s approval, through an innovative nasal spray[6], safely derived from… a party drug.[7]
Next, Compass Pathways (backed by ATAItai Life Sciences AG), received FDA breakthrough therapy designation[8] on a treatment derived from… magic mushrooms.[9]
ATAItai’s raised over US$100 million[10] to date from major investors such as PayPal co-founder Peter Thiel—and they’re currently seeking an USD$800 million valuation.[11]
Big league investment is taking notice of this potential psychedelics-led revolution in mental health, as Canopy Growth Founder Bruce Linton, and Shark Tank host Kevin O’Leary have put skin in the game into another leading psychedelic pharma company advancing multiple promising psychedelic medicine prospects.[12]
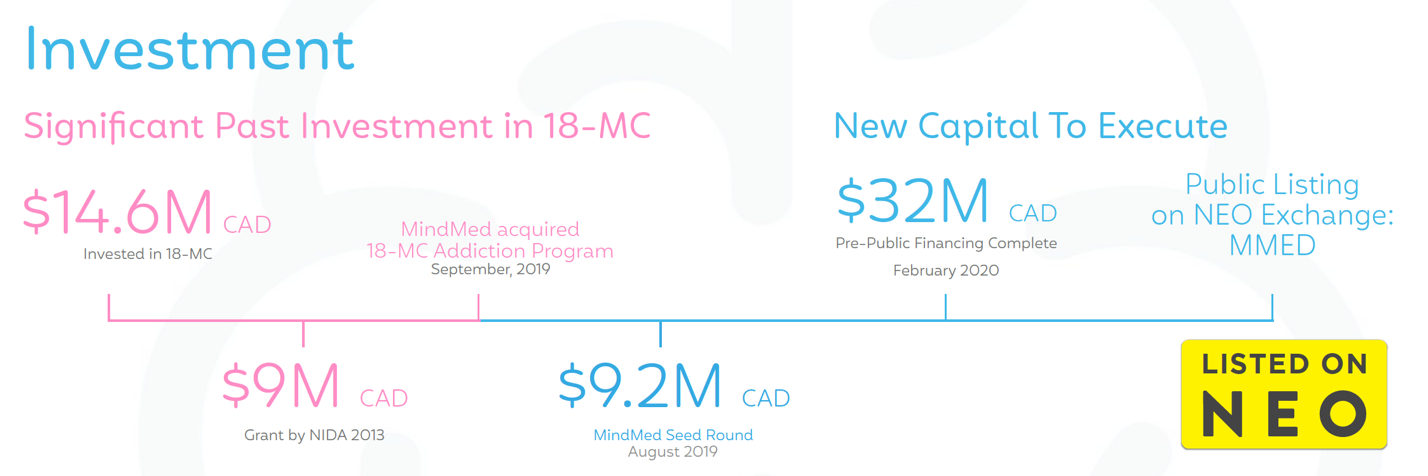
This little-known company we’ve highlighted has assembled a compelling IP portfolio, clinical pipeline, and drug development platform for psychedelics—raising approximately USD $40 million since launch.
Eight Capital, a leading investment bank in Canada, initiated the first ever equity research report of the psychedelics sector on MindMed. The report named 3 industry leaders : MindMed, Compass, and ATAI. MindMed is the ONLY publicly traded company of these 3 industry stalwarts.
And its listing truly hit the ground running…
On its first trading day on the NEO Exchange, this company broke the record for the most shares traded ever on a single day on the NEO. The company’s current market cap sits at CAD$152 million as of May 29, 2020, less than two months into trading.
The Magic of Mind Medicine (MindMed) Inc.
Mind Medicine (MindMed) Inc. (NEO: MMED) (OTCQB:MMEDF) (FRA:BGHM) is a next-gen neuro-pharmaceutical company that discovers, develops and deploys treatments derived from psychedelics to improve health, promote wellness and alleviate suffering.
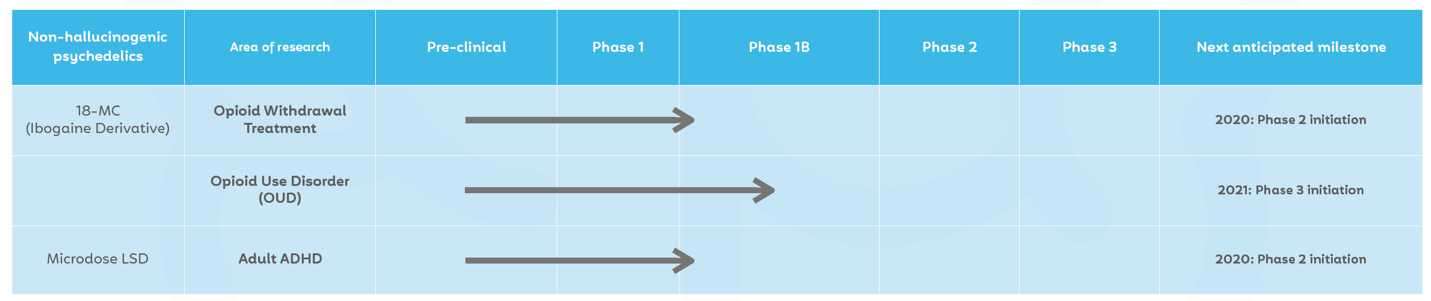
It’s clearly doing this by thinking outside the box—and it’s already preparing and soon starting Phase 2 clinical trials for two of its leading programs, each dealing with its own large, critical mental health addressable markets.

The first addresses the ongoing opioid epidemic that’s affecting millions, and has cost the U.S. economy trillions of dollars since it began. This crisis is only getting worse during COVID-19.
MindMed’s proprietary 18-MC program is developing a potential anti-addiction treatment derived from the Schedule I pharmaceutical, ibogaine—a hallucinogenic known to treat addictions since as early as 1962, but only now gaining traction when safely derived and administered.
The other treatment entering Phase 2 trials is MindMed’s Microdosing LSD program, that is evaluating if it can treat Adult ADHD.
Silicon Valley CEOs and developers have been using forms of this medication for years to improve their work efficiency. MindMed is undertaking to one day make LSD microdosing into a FDA approved medicine if the clinical trials prove it is effective.
Now the company has also recently announced[14] a third next-generation psychedelic therapy to its R&D pipeline—MDMA (the active ingredient in Ecstasy).
Mind Medicine (MindMed) Inc.’s (NEO: MMED) (OTCQB:MMEDF) (FRA:BGHM) full suite of drug development programs and R&D all have the potential to become industry game changers.
They are gaining all types of attention along the way, from mainstream media write-ups to heavyweight investors.
MEDICAL IBOGAINE FOR SOLVING THE OPIOID CRISIS
The opioid crisis is getting out of hand and the Whitehouse Council of Economic Advisors Estimate that the opioid crisis costs the US Economy $504 billion a year.[15]
Now with the current lockdown orders in place, and increased social isolation, the problem is only getting worse[16]. For many struggling with addiction, treatments and support systems have been disrupted—which may increase the risk of overdose deaths.
States are already seeing significant spikes in overdoses in the first half of this year, with an average 20% increase of overdose submissions since the first reported case that kicked off the pandemic lockdowns hit US shores.[17]
What does this all mean?
In short, psychiatric professionals will need more tools and treatment options—especially when they’re being forced to use telemedicine (diagnosis and prescriptions by phone), as they deal with everything from ADHD[18], to anxiety and addictions.[19]
In the News
- This New York City Pharma Startup Wants To Turn LSD Into An FDA-Approved Medicine For Anxiety Disorder – Forbes
- 3 Psychedelic Stocks to Buy for HUGE Gains in the Shroom Boom – Nasdaq
- Cannabis Countdown: Top 10 Marijuana And Psychedelic Stock News Stories Of The Week – Benzinga
- MindMed Lists on NEO to Become World’s First Psychedelic Pharmaceutical Company to Go Public – Financial Post
- Shark Tank’s Kevin O’Leary and the co-founder of the largest weed company just helped take a psychedelics startup public – Yahoo! Finance
MindMed Is Developing a Treatment for Addiction
“drug addiction is a disease of the brain”
– Dr. Nora Volkow, MD, Director, National Institute on Drug Abuse
Addiction is defined as a disease by most medical associations, including the American Medical Association and the American Society of Addiction Medicine.[20]
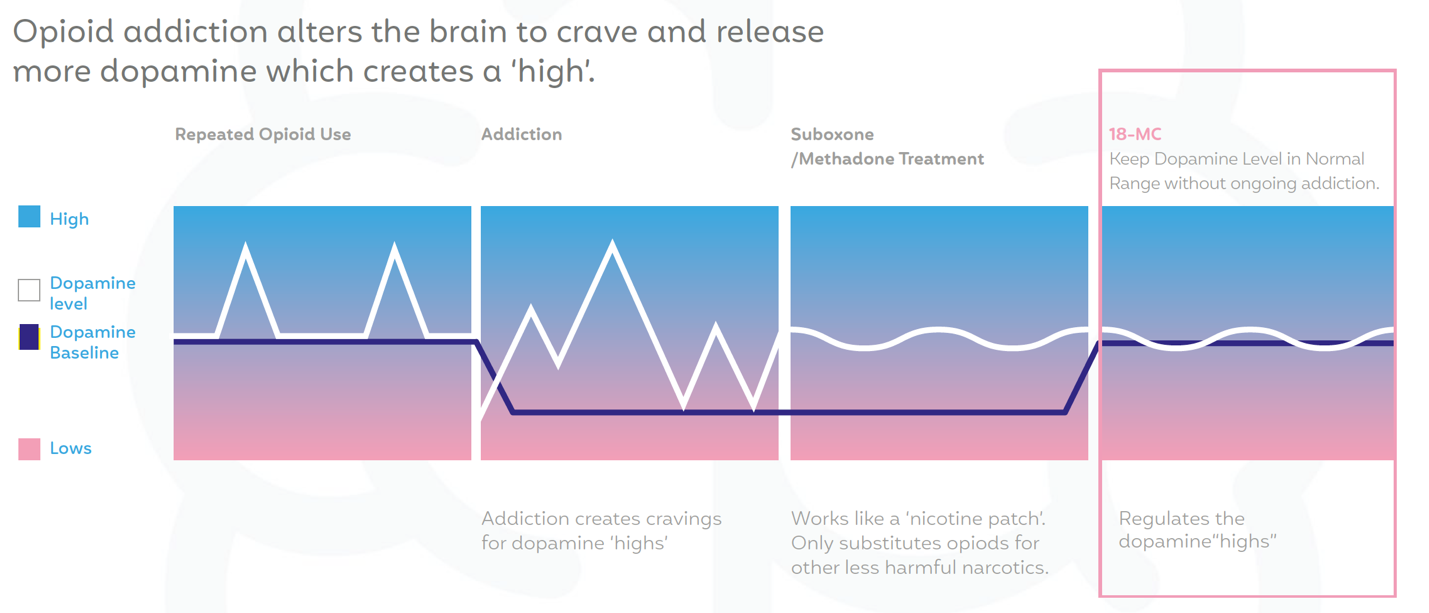
Existing medicines, such as methadone and suboxone, that are prescribed for addiction only act as a replacement therapy like the nicotine patch and these medicines are not treating Addiction as a brain disease. (Side note, the inventor of the nicotine patch Dr. Jed Rose is on the Scientific Advisory Board of MindMed).
The reality is that existing treatments just replace one harmful narcotic like heroin with one that is a little less harmful, but can also cause trauma. These treatments do not solve the core problem of addiction, as reflected by high relapse rates.
MindMed wants to actually solve addiction in a new way; not through a pill a day for the rest of one’s life. Instead, MindMed is working to develop treatments that could help patients transition back to a normal life, without relying on opioids and their associated dangers.
To treat this deadly disease, the 18-MC program, from Mind Medicine (MindMed) Inc. (NEO: MMED) (OTCQB:MMEDF) (FRA:BGHM) may finally be the treatment addiction sufferers have been waiting for.
MindMed is one of the first disruptive companies that intends to truly treat addictions as a brain disease.
18-MC is a synthetic derivative of the Schedule I drug, ibogaine—which itself comes from a root in West Africa called iboga, long used in religious rituals, and known for its visions.
But in 1962, ibogaine’s therapeutic efficacy against addictions was first witnessed[21], in particular when helping patients with detoxification from heroin.
The original discoverer of this treatment method believed his life’s work held great promise in helping over 164[22] million people around the world who are affected by substance use disorders.
However, ibogaine has some potential drawbacks, so MindMed decided to create its own synthetic version that did not have:
- Heart complications (inducing cardiac arrhythmias),[23][24]
- Hallucinations[25]
Enter 18-methoxycoronaridine (aka “18-MC”), which is a synthetic ibogaine derivative.
The potential of this new anti-addiction drug did not go unnoticed.
In 2012, the 18-MC program received a USD$6.8 million grant from the National Institute on Drug Abuse (NIDA).[26]NIDA is the leading US Government authority on drug addiction research in America. MindMed is aiming to develop methods to use 18-MC to fight addiction, substance use disorders and tackle the opioid crisis once and for all.
It eliminates the cardiac risk. It eliminates the hallucinations.
AND it is ADDRESSING the one of the most severe attributes of addiction, RELAPSE.
Ask any addict or alcoholic and they will tell you the hardest part of addiction is not getting sober, it’s staying sober. MindMed is trying to solve this problem. Solving for CRAVINGS could potentially crack the code on addiction.
MindMed intends for 18-MC to keep the patient’s dopamine levels in a normal range, without inducing ongoing addiction.
Most importantly, it has the potential to BOTH alleviate drug cravings and impede the relapse of drug use.
… and MindMed has the IP.
The addiction treatment market is worth a whopping $42 Billion a year[27] in the United States alone. This suggests that 100% of American’s have a drug addiction. The actual stat is “Almost 21 million Americans have at least one addiction, yet only 10% of them receive treatment.” There are 328 million people in America.[28]America is in desperate need of innovative solutions to all forms of addictions.
On April 16, 2020, MindMed announced it had initiated dosing its first patient with 18-MC in a Human Safety Study of Novel Treatment for Opioid Addiction.[29]
The company is planning to begin Phase 2 clinical trials by the end of 2020 that will help move 18-MC closer to a potential FDA approval—which would give MindMed the coveted first-to-market advantage that every breakthrough pharma company seeks.
MindMed may also have blockbuster drug potential if it can conduct additional trials for other addictions including :
- Alcoholism[30]
- Methamphetamine
- Cocaine
- Nicotine
SILICON VALLEY LEADING ‘MICRODOSING’ TO MAINSTREAM
Thanks to Hollywood depictions, psychedelics have since the 1960s been often associated with all types of wild hallucinogenic trips, filled with all types of colors, shapes, and imaginative designs.
But what’s lesser known is how psychedelics have strongly influenced much of the technological advances we enjoy today.

Apple visionary Steve Jobs was said to have partaken in psychedelics, having chided his rival Bill Gates for being “unimaginative” and recommending he should take some LSD. [31]
Today, many of the greatest minds in Silicon Valley are listening to the late Jobs’ advice, and boosting their careers with psychedelics, through the use of a method called Microdosing.
The concept involves taking tiny doses of psychedelics, often specks the size of crumbs, not to feel high, but to feel more focused, creative, and present.
And according to some intriguing research, it appears that microdoses may activate just the right amount of receptors in the brain for us to be our better selves.[32]
Amid a hyper hustle culture that’s ultra-competitive, professionals in Silicon Valley are seeking a mental edge that could mean the difference between becoming the next big billion-dollar company or the next dud. These white-collar professionals are shelling out thousands of dollars per month just to have a psychedelics guru lead them through microdosing their way to career clarity.[33]
And it’s not just these high-tech megastars that are seeking a boost from the microdosing trend—it’s even being reported as assisting stay-at-home moms get through the rigours of a typical day.[34]
By microdosing these drugs, the unwanted hallucinogenic effects are removed, while the more pleasant “sub-perceptual” (unnoticeable) effect remains.
But all these adventuristic “micro-doses” are currently carrying on these activities in an illegal manner, without the necessary research and fine-tuning to back it up.
Mind Medicine (MindMed) Inc. (NEO: MMED) (OTCQB:MMEDF) (FRA:BGHM) is looking to change that, by becoming the first ever exchange-traded psychedelics company to take microdosing into Phase 2 clinical trials for Adult ADHD.[35]
If they succeed in their goals of one day gaining FDA approval, their IP and first mover status has the potential to enable LSD ( aka “acid”) to be safely and effectively taken at home. Luckily, the company is already working on improving the safety of taking LSD through new technologies.
MindMed Acquires Exclusive License to Eight Clinical Trials of LSD, Partners with World-Leading Psychedelic Research Laboratory at University Hospital Basel
On April 1, MindMed announced the signing of a multi-year collaboration with the laboratory of Professor Dr. Matthias Liechti, the world-leading psychedelics pharmacology and clinical research group at University Hospital Basel in Switzerland.[36]
Through the collaboration, MindMed gains exclusive worldwide rights to data, compounds, and patent rights associated with the Liechti laboratory’s research with LSD and other psychedelic compounds.
Generated over the course of a decade, the data represents, according to Dr. Liechti, “the largest collection of clinical trials around LSD” that exists!
And MindMed now has the exclusive global rights to file patents and use the patents for it!
“This exclusive partnership puts MindMed at the forefront of efforts to develop LSD and other psychedelics while also setting the tempo and caliber of our growing IP portfolio and clinical trial pipeline.”
– Dr. Nora Volkow, MD, Director, National Institute on Drug Abuse
Right off the bat, MindMed will begin exploring the data regarding two potential applications for LSD:
- For the treatment of anxiety, and
- For the potential treatment of adult ADHD
The adult ADHD market is under-served because while 4.4%[37] of the general population have been officially diagnosed with the condition, studies have shown that over 20% of the entire adult population show symptoms of ADHD.[38]
Now coupling in the fact that almost all current ADHD medical treatments involve stimulants that cause a long list of unwanted symptoms, it’s unsurprising that almost half of all young adults stop taking their medications upon entering college.[39]
Add to that, over 50% of people with ADHD also suffer from Anxiety[40], this LSD data from University Hospital Basel is going to be pivotal in creating potentially disruptive medicines that may be able to treat a continuum of mental health illnesses.
Enhanced focus for college students can be crucial, much like for the Silicon Valley engineers.
But untreated ADHD is also strongly linked to conditions being exacerbated by the current health crisis, including low self-esteem, struggles with impulsiveness, higher emotional dysregulation, substance abuse, and an overall significantly lower quality of life.[41]
Adult ADHD affects 10 million adults in the US.[42] It is a MASSIVE market and it is ripe for disruption.
So by improving and refining the process, MindMed presents one of the market’s best hopes to help us be our better selves, through the use of microdosing psychedelics.
Next Gen Psychedelics R&D : Combining MDMA with Other Psychedelics
MindMed is also working on MDMA[43]. It recently added MDMA to its R&D portfolio and will be looking how it can combine MDMA and LSD into a next generation psychedelic therapy.
MindMed CEO JR Rahn said “We see an amazing opportunity to optimize the subjective effect profiles of known psychedelics like LSD and MDMA to potentially make a more advanced and comfortable therapeutic experience for the patient,”
THE MARKET’S TRIP DOWN THE RABBIT HOLE
While it’s true that Mind Medicine (MindMed) Inc. (NEO: MMED) (OTCQB:MMEDF) (FRA:BGHM) isn’t the only company in the psychedelics-therapy space, its multiple programs and progress through FDA clinical trial milestones make it one of the market’s top publicly-traded pure-play psychedelic contenders.
The company is currently preparing and will soon enter the Phase 2 trial stage for two of its programs, both of which it is working towards being the first-to-market title holder.
As evidenced by Eli Lilly and Company (NYSE: LLY) in the 90s, that first-to-market advantage led to billions of dollars in sales for a single antidepressant drug.
It’s the difference between being the household name, and just another alternative.
With its two platforms underway, MindMed is already poised to go, and having successfully raised approximately USD $40 million since launch.

MindMed has attracted the backing of big league investors, including “Mr. Wonderful” Kevin O’Leary (host of Shark Tank), and Canopy Growth Founder and Former CEO, Bruce Linton.
At March 31, 2020, MindMed’s cash on hand was USD $20.5 million respectively.[44]
Following that, the company raised approximately CAD $13.2 million through a bought deal offering with Eight Capital.[45]
MindMed’s highlights further establish how the market is responding particularly well to this wave of psychedelic-based medicines. Other success stories in the space include:
- Champignon Brands (CSE: SHRM) (OTCPK: SHRMF) recently went on a major run—gaining 140.4% in just two days.
- Compass Pathways secured $80 million[46] in financing to boost its depression clinical trial based on psilocybin or “magic mushrooms”
- Atai Life Sciences AG (largest shareholder of Compass Pathways) is seeking a valuation of $800 million[47] after recently raising $25 million
Amid these comparables, MindMed has more than kept pace, by meeting its milestones and adding new programs, IP and clinical trials acquisitions, successfully raising funds, and capturing the public’s attention with mainstream media coverage.
“Through psychedelic inspired medicines, MindMed has the potential to treat the most challenging and damaging mental health issues of our time.”
– James Bailey, Bail Capital
“There’s probably untapped value, which will only go to the people who are at the beginning and bold.”
– Bruce Linton, Founder and Former CEO of Canopy Growth
“This could save lives, cure depression, help alcoholism, get people off opioids—why wouldn’t I want to be invested?”
– Kevin O’Leary, Host of Shark Tank and Venture Capitalist
PIPELINE CATALYSTS

MindMed’s Management Team and Advisory Board
 JR Rahn – Founder & Co-CEO. Rahn is a former Silicon Valley tech executive who realized that transformational solutions to mental illness and addiction might lie in psychedelic medicines. After 2 years of his own research and investment in psychedelic research, he partnered with drug development veteran Stephen Hurst to start Mindmed in 2019. Before starting Mindmed, Rahn worked in market expansion and operations at Uber, after which he was backed by the Silicon Valley tech accelerator Y Combinator for his company Upgraded, which partnered with Apple to provide device financing for Apple customers in Europe.
JR Rahn – Founder & Co-CEO. Rahn is a former Silicon Valley tech executive who realized that transformational solutions to mental illness and addiction might lie in psychedelic medicines. After 2 years of his own research and investment in psychedelic research, he partnered with drug development veteran Stephen Hurst to start Mindmed in 2019. Before starting Mindmed, Rahn worked in market expansion and operations at Uber, after which he was backed by the Silicon Valley tech accelerator Y Combinator for his company Upgraded, which partnered with Apple to provide device financing for Apple customers in Europe.
 Stephen L. Hurst, JD – Founder & Co-CEO. Hurst has 35+ years’ experience in the biopharmaceutical industry, including work for The Immune Tolerance Institute, The Regents of the University of California, The World Bank and BIO Ventures for Global Health. He’s previously advised on psychedelic therapies research and clinical trial designs. He’s also held senior executive positions with Sequential and Inhale Therapeutic Systems (now Nektar Therapeutics, Inc.), where he helped the company grow to over 700 employees and raised +$700 million in investment capital. Steve and Drs. Freeman and Glick co-founded Savant HWP, Inc., in 2009 to advance 18-MC to clinical trials.
Stephen L. Hurst, JD – Founder & Co-CEO. Hurst has 35+ years’ experience in the biopharmaceutical industry, including work for The Immune Tolerance Institute, The Regents of the University of California, The World Bank and BIO Ventures for Global Health. He’s previously advised on psychedelic therapies research and clinical trial designs. He’s also held senior executive positions with Sequential and Inhale Therapeutic Systems (now Nektar Therapeutics, Inc.), where he helped the company grow to over 700 employees and raised +$700 million in investment capital. Steve and Drs. Freeman and Glick co-founded Savant HWP, Inc., in 2009 to advance 18-MC to clinical trials.
 Scott Freeman, MD – President and Chief Medical Officer. Dr. Freeman has extensive experience in clinical trial work and FDA regulatory strategy. As the CMO of Savant he was the primary lead behind the National Institutes of Health (NIH) grant in bringing 18-MC to IND and its first-in-human clinical trial. He served as VP of Clinical Development at Onyx Pharmaceutical and was head of both the clinical development and operations groups, which lead to FDA approval of Nexavar for kidney and liver cancer. He was an Associate Professor at Tulane University and guest researcher at the NIH in basic and clinical research.
Scott Freeman, MD – President and Chief Medical Officer. Dr. Freeman has extensive experience in clinical trial work and FDA regulatory strategy. As the CMO of Savant he was the primary lead behind the National Institutes of Health (NIH) grant in bringing 18-MC to IND and its first-in-human clinical trial. He served as VP of Clinical Development at Onyx Pharmaceutical and was head of both the clinical development and operations groups, which lead to FDA approval of Nexavar for kidney and liver cancer. He was an Associate Professor at Tulane University and guest researcher at the NIH in basic and clinical research.
 Donald Gehlert, PhD – Chief Scientific Officer. Dr. Gehlert has extensive experience in drug discovery and expertise in key functional areas of exploratory development and disease biology. During his career at Lilly, he led or participated in teams that introduced 19 molecules into the Lilly pipeline including both small and large molecule therapies. He also participated on Phase I and II clinical development teams that designed and delivered translational proof of concept studies in the areas of ADHD, obesity, AUD, depression, pain and migraine. He’s a co-author on 182 publications and a co-inventor on 15 issued and pending patents.
Donald Gehlert, PhD – Chief Scientific Officer. Dr. Gehlert has extensive experience in drug discovery and expertise in key functional areas of exploratory development and disease biology. During his career at Lilly, he led or participated in teams that introduced 19 molecules into the Lilly pipeline including both small and large molecule therapies. He also participated on Phase I and II clinical development teams that designed and delivered translational proof of concept studies in the areas of ADHD, obesity, AUD, depression, pain and migraine. He’s a co-author on 182 publications and a co-inventor on 15 issued and pending patents.
 Carol Nast – Chief Operating Officer. Nast has spent her career in executive level positions with large multinational companies and early stage companies in the medical industry. She’s a recognized expert in product development and commercialization and is experienced in the management of complex, multinational partner programs and has successfully led the development and commercialization of 100+ products. She was COO at NuGen, a genomics company, and served in executive level positions at Inhale Therapeutics (Nektar), Syva (a division of Syntex Pharmaceuticals,) BioRad and Pfizer.
Carol Nast – Chief Operating Officer. Nast has spent her career in executive level positions with large multinational companies and early stage companies in the medical industry. She’s a recognized expert in product development and commercialization and is experienced in the management of complex, multinational partner programs and has successfully led the development and commercialization of 100+ products. She was COO at NuGen, a genomics company, and served in executive level positions at Inhale Therapeutics (Nektar), Syva (a division of Syntex Pharmaceuticals,) BioRad and Pfizer.
 Jeanne Bonelle – Executive Vice President, Global Quality. Bonelle has 30+ years’ experience developing quality organizations within startup firms and transitioning to commercial operations. Her last four positions were to establish quality systems within the developmental phase for a wide range of products, including Senior Director of Quality Assurance at Inhale Therapeutic Systems (now Nektar Therapeutics); Director of Quality Assurance at Cholestech (now Alere); Manager of Quality Assurance at BioTrack, (now a subsidiary of Roche Diagnostics GmbH); Manager of Quality Assurance at BioResponse (now Baxter HealthCare).
Jeanne Bonelle – Executive Vice President, Global Quality. Bonelle has 30+ years’ experience developing quality organizations within startup firms and transitioning to commercial operations. Her last four positions were to establish quality systems within the developmental phase for a wide range of products, including Senior Director of Quality Assurance at Inhale Therapeutic Systems (now Nektar Therapeutics); Director of Quality Assurance at Cholestech (now Alere); Manager of Quality Assurance at BioTrack, (now a subsidiary of Roche Diagnostics GmbH); Manager of Quality Assurance at BioResponse (now Baxter HealthCare).
 Bruce Linton – Director. Founder and Former CEO and Chairman of Canopy Growth, Linton brings a wealth of experience in building strong tech-driven companies and developing world-class teams. He chairs MindMed’s Board’s Compensation, Governance and Nomination Committee. He’s currently Executive Chairman of GAGE Co., Special Advisor with Better Choice Company, and an Activist Investor with SLANG Worldwide, and OG DNA Genetics Inc., which has built and curated a seasoned genetic library and developed proven standard operating procedures for genetic selection, breeding, and cultivation.
Bruce Linton – Director. Founder and Former CEO and Chairman of Canopy Growth, Linton brings a wealth of experience in building strong tech-driven companies and developing world-class teams. He chairs MindMed’s Board’s Compensation, Governance and Nomination Committee. He’s currently Executive Chairman of GAGE Co., Special Advisor with Better Choice Company, and an Activist Investor with SLANG Worldwide, and OG DNA Genetics Inc., which has built and curated a seasoned genetic library and developed proven standard operating procedures for genetic selection, breeding, and cultivation.
 Brigid Makes – Director. Prior to serving as an independent consultant for private medical device companies, Makes served as Senior VP and CFO of Miramar Labs, which was acquired by Sientra in July 2017. From 2006 to 2011 she served in the same roles for AGA Medical (acquired by St. Jude Medical). Prior to AGA Medical, she served in a variety of executive positions, including as CFO, for Nektar Therapeutics (formerly Inhale Therapeutics). She also served as CFO for Oravax, from 1998 to 1999 and Haemonetics Corp, from 1995 to 1998. Ms. Makes chairs the Board’s Audit Committee.
Brigid Makes – Director. Prior to serving as an independent consultant for private medical device companies, Makes served as Senior VP and CFO of Miramar Labs, which was acquired by Sientra in July 2017. From 2006 to 2011 she served in the same roles for AGA Medical (acquired by St. Jude Medical). Prior to AGA Medical, she served in a variety of executive positions, including as CFO, for Nektar Therapeutics (formerly Inhale Therapeutics). She also served as CFO for Oravax, from 1998 to 1999 and Haemonetics Corp, from 1995 to 1998. Ms. Makes chairs the Board’s Audit Committee.
 Stanley D. Glick, PhD, MD – Director, Chair of Scientific Advisory Board, and Senior Clinical Advisor. Dr. Glick is a former professor of pharmacology at Mount Sinai School of Medicine, and once chaired the pharmacology and neuroscience program at Albany Medical College. Funded by the National Institute on Drug Abuse (NIDA) for 40 years, his major research focused on the neurobiology of drug addiction. Dr. Glick is a co-inventor of a novel group of agents for treating drug addiction, including 18-methoxycoronaridine (18-MC). He’s authored or co-authored 450+ experimental papers, reviews and abstracts, and has served on journal editorial boards and NIH advisory committees.
Stanley D. Glick, PhD, MD – Director, Chair of Scientific Advisory Board, and Senior Clinical Advisor. Dr. Glick is a former professor of pharmacology at Mount Sinai School of Medicine, and once chaired the pharmacology and neuroscience program at Albany Medical College. Funded by the National Institute on Drug Abuse (NIDA) for 40 years, his major research focused on the neurobiology of drug addiction. Dr. Glick is a co-inventor of a novel group of agents for treating drug addiction, including 18-methoxycoronaridine (18-MC). He’s authored or co-authored 450+ experimental papers, reviews and abstracts, and has served on journal editorial boards and NIH advisory committees.
 Matthew W. Johnson, Ph.D – Advisory Board. Psychedelics and addictions expert Dr. Johnson is a Professor at Johns Hopkins, current President of the International Society for Research on Psychedelics, and former President of the American Psychological Association Psychopharmacology Division. He’s published ~50 scientific papers on psychedelics, the largest study of psilocybin in cancer distress, research on psychedelic treatment of tobacco addiction, and helped resurrect psychedelic research by writing psychedelic safety guidelines. His 2018 psilocybin review recommended Schedule IV upon medical approval.
Matthew W. Johnson, Ph.D – Advisory Board. Psychedelics and addictions expert Dr. Johnson is a Professor at Johns Hopkins, current President of the International Society for Research on Psychedelics, and former President of the American Psychological Association Psychopharmacology Division. He’s published ~50 scientific papers on psychedelics, the largest study of psilocybin in cancer distress, research on psychedelic treatment of tobacco addiction, and helped resurrect psychedelic research by writing psychedelic safety guidelines. His 2018 psilocybin review recommended Schedule IV upon medical approval.
Investment highlights for Mind Medicine (MindMed) Inc. (NEO: MMED) (OTCQB:MMEDF) (FRA:BGHM):
- MindMed is a pure-play psychedelics medicine company soon entering Phase 2 Clinical Trials for not one, but two psychedelics programs: 18-MC (Ibogaine Derivative) for opioid withdrawal treatment, and Microdose LSD for Adult ADHD.
- 18-MC is an anti-addiction treatment seeking to address an ongoing opioid crisis that costs American society $504 billion annually.[48]
- MindMed is the first ever exchange-traded psychedelics company to undertake Phase 2 trials of Microdosing LSD—a treatment growing in popularity among some of the top minds in Silicon Valley.
- Currently co-developing a new LSD Neutralizer Technology and if the patent filing is accepted will hold exclusive, worldwide rights ato shorten and even stop LSD trips—ie. an LSD ‘off-switch’ through this tech.
- Currently financially healthy position with cash on hand of $20.5 million respectively at March 31, 2020, and an additional $13.22 million CAD raised through a bought deal offering with Eight Capital on May 26, 2020.
- Support from high-profile investors, and scientific advisory board members, including Canopy Growth Founder and Former CEO Bruce Linton, Shark Tank host Kevin O’Leary, 18-MC inventor and leading addictions researcher Dr. Stanley D. Glick MD and leading psychedelics researcher Dr. Matthew W. Johnson of Johns Hopkins University.
- Management team and board with an extensive amount of industry experience attached to a variety of companies and approved drugs, including Lilly, Nektar, BVGH, Strattera, NuGen, Onyx, Roche, Pfizer, Nexavar, Allelix, SGC, Y Combinator, and Uber.
- https://www.who.int/medicines/publications/essentialmedicines/en/
- https://www.brucekalexander.com/pdf/Rat%20Park%201981%20PB&B.pdf
- IQVIA
- https://www.nytimes.com/2020/05/28/business/unemployment-stock-market-coronavirus.html
- https://www.livescience.com/psilocybin-depression-breakthrough-therapy.html
- https://www.forbes.com/sites/elliekincaid/2019/03/05/fda-approves-johnson–johnsons-ketamine-derived-drug-for-treatment-resistant-depression/#46851bc33e03
- https://pubmed.ncbi.nlm.nih.gov/17929712/
- https://compasspathways.com/compass-pathways-receives-fda-breakthrough-therapy-designation-for-psilocybin-therapy-for-treatment-resistant-depression/
- https://www.medicalnewstoday.com/articles/308850
- https://www.businessinsider.in/finance/news/atai-has-raised-100-million-to-turn-psychedelics-into-treatments-for-depression-and-anxiety-now-the-unusual-biotech-is-gearing-up-to-raise-millions-more-for-the-next-phase-of-human-testing-/articleshow/74346638.cms
- https://www.bnnbloomberg.ca/ipo-beckons-for-magic-mushroom-research-into-depression-1.1146829
- https://www.businessinsider.com/how-much-top-psychedelics-startups-have-raised-atai-compass-mindmed-2020-3
- https://www.ibogainealliance.org/about/howard-lotsof/
- https://www.mindmed.co/press-releases/mindmed-adds-mdma-to-develop-next-gen-psychedelic-therapies
- https://www.whitehouse.gov/sites/whitehouse.gov/files/images/The%20Underestimated%20Cost%20of%20the%20Opioid%20Crisis.pdf
- https://www.health.harvard.edu/blog/a-tale-of-two-epidemics-when-covid-19-and-opioid-addiction-collide-2020042019569
- https://files.constantcontact.com/a923b952701/dbf0b5a5-f730-4a6f-a786-47097f1eea78.pdf
- https://www.psychiatrictimes.com/coronavirus/best-practices-using-telemedicine-adhd-during-covid-19-pandemic
- https://www.syracuse.com/coronavirus/2020/05/telemedicine-use-by-upstate-new-yorkers-explodes-during-pandemic.html
- https://www.centeronaddiction.org/what-addiction/addiction-disease
- https://www.ibogainealliance.org/about/howard-lotsof/
- Moustafa,A.A.(2020).Cognitive,Clinical,andNeuralAspectsofDrugAddiction.UnitedKingdom:ElsevierScience.
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4382526/
- https://pubmed.ncbi.nlm.nih.gov/26807959/
- https://pubmed.ncbi.nlm.nih.gov/30216039/
- https://www.thefix.com/content/anti-addiction-drug-18-mc-begins-human-trials
- AddictionRehabIndustry:MarketResearchReport,January2020,MarketdataEnterprises,Inc.
- https://www.addictioncenter.com/addiction/addiction-statistics/
- https://www.mindmed.co/press-releases/mindmed-initiates-dosing-in-human-safety-study-of-novel-treatment-for-opioid-addiction-18-mc
- https://www.ucsf.edu/news/2005/01/5247/controversial-drug-shown-act-brain-protein-cut-alcohol-use
- https://www.forbes.com/sites/jackkelly/2020/01/17/silicon-valley-is-micro-dosing-magic-mushrooms-to-boost-their-careers/#17cbf0335822
- https://www.scientificamerican.com/article/do-microdoses-of-lsd-change-your-mind/
- https://www.bbc.com/worklife/article/20200106-can-drugs-help-you-choose-a-new-career
- https://www.theguardian.com/science/2019/may/03/psychedelic-drugs-women-taking-tiny-doses-hattie-garlick
- https://www.mindmed.co/news-posts/mind-medicine-becomes-1st-exchange-traded-psychedelics-company-to-begin-phase-2a-trials
- https://www.mindmed.co/press-releases/mindmed-acquires-exclusive-license-to-eight-clinical-trials-of-lsd-partners-with-world-leading-psychedelic-research-laboratory-at-university-hospital-basel
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2518387/
- https://pubmed.ncbi.nlm.nih.gov/27680974/
- https://www.additudemag.com/medication-adherence-adhd-college/
- https://adaa.org/understanding-anxiety/related-illnesses/other-related-conditions/adult-adhd
- https://pubmed.ncbi.nlm.nih.gov/27680974/
- ChildrenandAdultswithAttention-Deficit/HyperactivityDisorder(CHADD),
- https://www.google.com/url?q=https://www.benzinga.com/markets/cannabis/20/05/16122345/mindmed-to-include-mdma-to-research-portfolio&sa=D&ust=1590797339161000&usg=AFQjCNH5k4SGsc_z7rPl7tnujdTVRP4fig
- https://www.mindmed.co/press-releases/mindmed-reports-first-quarter-2020-results-and-corporate-update
- https://www.mindmed.co/press-releases/mindmed-announces-increase-in-bought-deal-offering-to-approximately-11-5-million
- https://news.crunchbase.com/news/compass-pathways-secures-80m-series-b-to-boost-depression-clinical-trial/
- https://www.bnnbloomberg.ca/ipo-beckons-for-magic-mushroom-research-into-depression-1.1146829
- https://www.whitehouse.gov/briefings-statements/cea-report-underestimated-cost-opioid-crisis/